Pioneering solutions, saving lives
Research is critical to combatting brain disease and improving the lives of those impacted, today and in the future.
Conducted by The Royal Melbourne Hospital’s Departments of Neurology and Neurosurgery, the active research programs and clinical trials supported by the Foundation cover an extensive breadth of neurological conditions.
Patients wishing to participate are encouraged to view the relevant trial and contact the trial co-ordinator for more information.
| Brain Tumour / Neurosurgery: | NsurgClerk@mh.org.au |
| Dementia: | DementiaTrials@mh.org.au |
| Epilepsy: | EpilepsyCoordinator@mh.org.au |
| Huntington’s Disease: | RMHHuntingtonDiseaseStudies@mh.org.au |
| Movement Disorders: | PDNurse@mh.org.au |
| Multiple Sclerosis: | MSClinic@mh.org.au |
| Neurophysiology: | RMH-ClinicalNeurophysiology@mh.org.au |
| Stroke: | RMH-StrokeResearchTeam@mh.org.au |
| General: | Neuro.Foundation@mh.org.au |
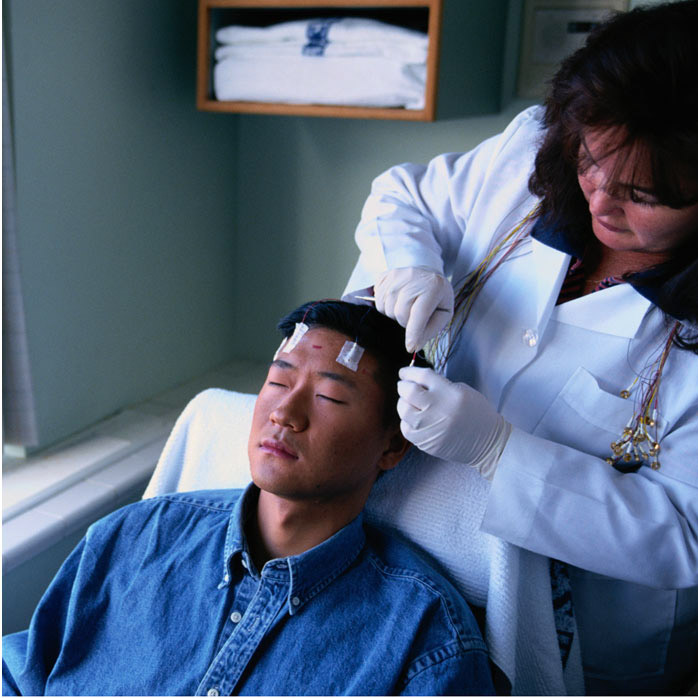
Seizure Detection and Classification Using Eyelid Movements: A Video-EEG Monitoring Study

Prevention of post-stroke epilepsy in patients with acute ischaemic stroke at high risk of developing seizures

Study on automated seizure detection via combined analysis of video and EEG clinical recordings

Clinical utility and cost-effectiveness of immediate vs delayed genome sequencing for refractory epilepsy in children and adults: a multicentre randomised controlled trial

PGx Identifying genetic and molecular biomarkers of epilepsy and the response to treatment