Pioneering solutions, saving lives
Research is critical to combatting brain disease and improving the lives of those impacted, today and in the future.
Conducted by The Royal Melbourne Hospital’s Departments of Neurology and Neurosurgery, the active research programs and clinical trials supported by the Foundation cover an extensive breadth of neurological conditions.
Patients wishing to participate are encouraged to view the relevant trial and contact the trial co-ordinator for more information.
| Brain Tumour / Neurosurgery: | NsurgClerk@mh.org.au |
| Dementia: | DementiaTrials@mh.org.au |
| Epilepsy: | EpilepsyCoordinator@mh.org.au |
| Huntington’s Disease: | RMHHuntingtonDiseaseStudies@mh.org.au |
| Movement Disorders: | PDNurse@mh.org.au |
| Multiple Sclerosis: | MSClinic@mh.org.au |
| Neurophysiology: | RMH-ClinicalNeurophysiology@mh.org.au |
| Stroke: | RMH-StrokeResearchTeam@mh.org.au |
| General: | Neuro.Foundation@mh.org.au |
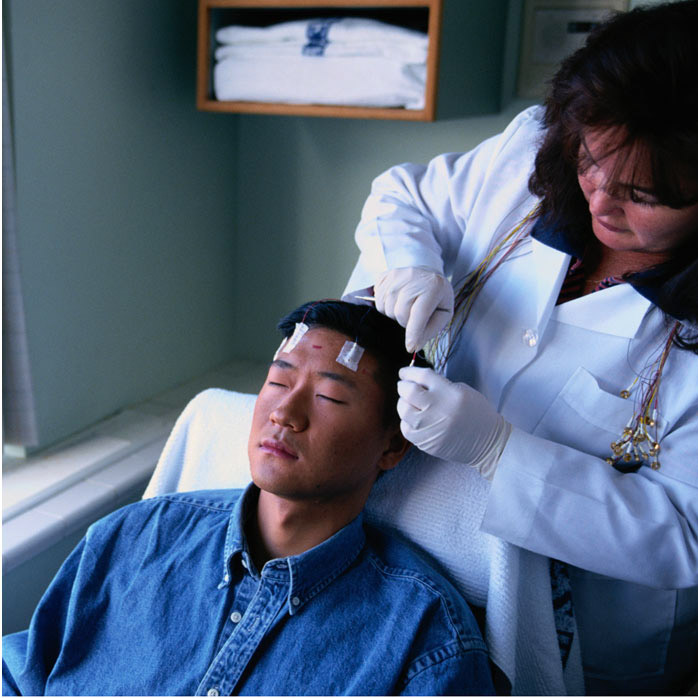
The Australian Pregnancy Register for Women on Antiepileptic Medication with Epilepsy or Allied Conditions : ERM15983

Long-term monitoring for cardiac arrhythmias in patients with drug resistant epilepsy

Utilising Prescription Patterns to Identify Increased Risk of Death After Diagnosis of Epilepsy

Sleep and Neuropsychiatric Comorbidities in Epilepsy

Psychological and cultural aspects of psychogenic non-epileptic seizures (PNES)